#CovidBoosters
Health & Fitness | Covid-19 News
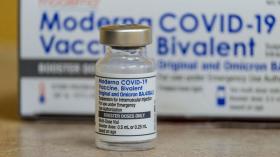
FDA vaccine advisers ‘disappointed’ and ‘angry’ that early data about new Covid-19 booster shot wasn’t presented for review last year
Some vaccine advisers to the federal government say they're "disappointed" and "angry" that government scientists and the pharmaceutical company Moderna didn't present a set of infection data on the company's new Covid-19 booster during meetings last year
News | In The News
Without Citing Clinical Trials, FDA Approves Emergency Use Of COVID-19 Boosters For Children 12 And Up
The Food and Drug Administration (FDA) announced that COVID-19 boosters for children between the ages of 12 and 15 have been approved for emergency authorized use based on “real-world” data from Israel.